What is colorectal cancer (CRC)?
CRC was the third commonest cancer in Hong Kong, and ranked second among the major causes of cancer deaths with a total of 2266 patients died from CRC in 2023, which accounted for 15.2% of cancer deaths1. The median age of CRC incidence was 69 years old2, and a continuously rising incidence in Hong Kong among individuals aged 55 or younger was observed3. With reference to the American Cancer Society Colorectal Cancer Screening Guideline in 2018, most people should begin screening for CRC soon after turning 454. While individuals who are at increased risk due to a family history of CRC, hereditary bowel syndrome or other known risk factors should undergo regular screening earlier5.
Risk factors for CRC5,6
| Unhealthy lifestyle (e.g. lack of exercise, high intake of red meat and processed meat, low fibre intake, alcohol consumption and smoking) |
| Family history of hereditary bowel syndrome (e.g. Lynch syndrome and familial adenomatous polyposis) |
| Family history of CRC in *first-degree relatives aged 60 or younger |
| Family history of CRC in more than one *first-degree relatives regardless of age at diagnosis |
| History of colonic polyps |
| Persistent inflammation of the bowel (e.g. ulcerative colitis) |
*first-degree relative: parents, siblings or children
Symptom
Early CRC can be asymptomatic7. Common symptoms of CRC include6,7:
| Blood or mucus in the stool, or rectal bleeding |
| A change in bowel habits, such as persistent constipation or diarrhoea, or narrowing of the stool |
| A feeling of incomplete bowel emptying |
| Unexplained weight loss |
| Abdominal pain, bloating or cramping |
| Symptoms of anaemia (such as fatigue and shortness of breath) |
Screening method
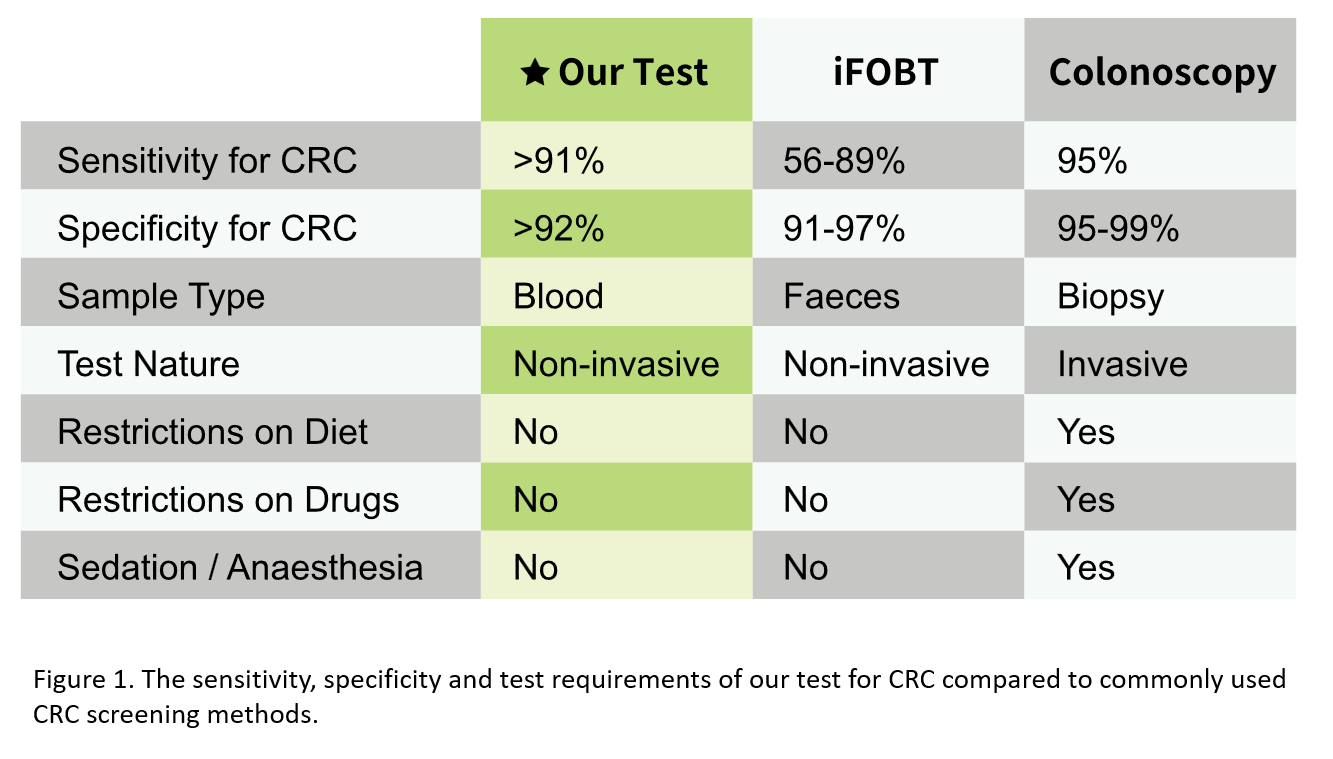
Traditional CRC screening tests include faecal occult blood testing (FOBT) and colonoscopy. FOBT can be either guaiac-based (gFOBT) or immunochemical (iFOBT / FIT), with iFOBT considered an improved version due to having no dietary restrictions and its higher sensitivity for detecting CRC8,9. However, FOBT may give false positive results in individuals experiencing bleeding other than CRC or polyps, such as a stomach ulcer or a haemorrhoid9. Moreover, FOBT may also give false negative results in individuals having CRC or polyps that do not bleed9.
The Hong Kong Government has launched the Colorectal Cancer Screening Programme since 2020 and has provided free CRC screening services using iFOBT for Hong Kong residents aged 50 to 75 who are suitable for colonoscopy, with subsidised follow-up colonoscopy if the result is positive. As of the end of 2024, over 510000 eligible individuals had participated in this programme, with approximately 77000 participants received positive iFOBT results. Among iFOBT-positive participants who received a colonoscopy, about 3400 individuals were diagnosed with CRC while approximately 40000 cases of colorectal adenoma (a type of polyp that can turn into cancer) were identified10. Therefore, a large portion of the iFOBT-positive individuals without any polyps or CRC received invasive and uncomfortable follow-up colonoscopy, which is a procedure requiring bowel preparation and carrying risks such as colonoscopic perforation and gastrointestinal bleeding11. In addition, the iFOBT-positive individuals might also experience psychological distress from the positive results12.
Colorectal polyps grow slowly and usually take about 10 years to become cancerous13. Early CRC can be asymptomatic7, a large number of CRC cases were diagnosed at advanced stages (Stage III and IV)2 where treatment options are limited. When detected early, colorectal cancer is a very treatable and curable form of cancer6. Therefore, there is a need for a non-invasive and user-friendly screening method to detect CRC at early stages accurately.
Early Colorectal Cancer Genetic Biomarker Screening Test (Blood-based)
This innovative test detects CRC-specific methylated DNA fragments in plasma which are highly associated with the pathogenesis of CRC14 through methylation-sensitive fluorescence quantitative PCR (qPCR). It allows more effective treatment when CRC is detected early, which is especially important for individuals with an increased risk of CRC.
Why choose our test?
Non-invasive and convenient
| Uses a simple blood sample |
Minimizing the use of unnecessary and invasive procedures
| Can be used as a triage before colonoscopy |
Highly sensitive detection
| High sensitivity for detecting CRC at early stages (Stage I and II, 87.4%) and CRC at all stages (91.2%)15 |
Validated in clinical studies
| Has been validated using 1194 blood samples15, ensuring high accuracy and reliability |
CE-IVD certified and NMPA approved
Test specifications
| Test code | Methodology | Sample requirement | Turnaround time |
|---|---|---|---|
| OCR | Real-time PCR | 10ml cfDNA Blood Tube (Sample should be stored at room temperature) | 10 working days |
References
- 1 Centre for Health Protection, Department of Health - Colorectal Cancer. www.chp.gov.hk/en/healthtopics/content/25/51.html
- 2 Hospital Authority. Colorectal Cancer in 2022. 2024, www3.ha.org.hk/cancereg/pdf/factsheet/2022/colorectum_2022.pdf
- 3 Press Releases - News - Faculty of Medicine, the Chinese University of Hong Kong. 12 June 2018, www.med.cuhk.edu.hk/press-releases/cuhk-study-sees-increasing-global-incidence-of-colorectal-cancer-among-younger-people
- 4 Wolf, Andrew M. D., et al. “Colorectal Cancer Screening for Average‐risk Adults: 2018 Guideline Update From the American Cancer Society.” CA a Cancer Journal for Clinicians, vol. 68, no. 4, May 2018, pp. 250–81. https://doi.org/10.3322/caac.21457
- 5 Recommendations of the Cancer Expert Working Group (CEWG) on Cancer Prevention and Screening | Prevent Colorectal Cancer. www.colonscreen.gov.hk/en/public/about_crc/recommendation_of_the_cancer_expert_working_group_on_cancer_prevention_and_screening.html
- 6 Cancer Online Resource Hub - Cancers in Hong Kong - Common Cancers in Hong Kong - Colorectal Cancer. www.cancer.gov.hk/en/hong_kong_cancer/common_cancers_in_hong_kong/colorectal_cancer.html
- 7 Common Symptoms | Prevent Colorectal Cancer. www.colonscreen.gov.hk/en/public/about_crc/common_symptoms.html
- 8 Fecal Occult Blood Test (FOBT). medlineplus.gov/lab-tests/fecal-occult-blood-test-fobt.
- 9 Fecal Occult Blood Test - Mayo Clinic. www.mayoclinic.org/tests-procedures/fecal-occult-blood-test/about/pac-20394112
- 10 Cancer Expert Working Group on Cancer Prevention and Screening, et al. “Colorectal Cancer Screening and Prevention.” Non-Communicable Diseases Watch, Mar. 2025, www.chp.gov.hk/files/pdf/ncd_watch_mar_2025_en.pdf
- 11 Ko, Cynthia W., and Jason A. Dominitz. “Complications of Colonoscopy: Magnitude and Management.” Gastrointestinal Endoscopy Clinics of North America, vol. 20, no. 4, Oct. 2010, pp. 659–71. https://doi.org/10.1016/j.giec.2010.07.005
- 12 Denters, M. J., et al. “FIT False-positives in Colorectal Cancer Screening Experience Psychological Distress up to 6 Weeks After Colonoscopy.” Supportive Care in Cancer, vol. 21, no. 10, May 2013, pp. 2809–15. https://doi.org/10.1007/s00520-013-1867-7
- 13 “Colorectal (Colon) Cancer.” Cleveland Clinic, 5 May 2025, my.clevelandclinic.org/health/diseases/14501-colorectal-colon-cancer
- 14 Barault, Ludovic, et al. “Discovery of Methylated Circulating DNA Biomarkers for Comprehensive Non-invasive Monitoring of Treatment Response in Metastatic Colorectal Cancer.” Gut, vol. 67, no. 11, Oct. 2017, pp. 1995–2005. https://doi.org/10.1136/gutjnl-2016-313372
- 15 Wang, Zhijie, et al. “Evaluation of a Plasma Cell-free DNA Methylation Test for Colorectal Cancer Diagnosis: A Multicenter Clinical Study.” BMC Medicine, vol. 22, no. 1, Oct. 2024, https://doi.org/10.1186/s12916-024-03662-y
- 16 Bretthauer, M. “Colorectal Cancer Screening.” Journal of Internal Medicine, vol. 270, no. 2, May 2011, pp. 87–98. https://doi.org/10.1111/j.1365-2796.2011.02399.x
- 17 “Colonoscopy Diet Advice and Bowel Preparation - Overview.” Guy’s and St Thomas’ NHS Foundation Trust, www.guysandstthomas.nhs.uk/health-information/colonoscopy-diet-advice-and-bowel-preparation
- 18 Common Screening Tests | Prevent Colorectal Cancer. www.colonscreen.gov.hk/en/public/about_crc/common_tests_for_crc_screening.html