We’re all about building
a healthier society
We believe that DNA testing can empower healthcare professionals and their patients. We understand the concerns that surround inherited genetic illness, cancer and serious health conditions and work to mitigate the risks and develop more targeted solutions. Our genetic screening and advisory services apply the latest technology and data analysis to assist in doctors and specialists providing effective treatments options.
Our Vision
We aim to be a leading global innovator in the biotechnology industry by continuously developing ground-breaking molecular diagnostic (MDx) technologies, increasing health awareness in society and inspiring future generations in order to achieve a healthier community with higher standard of living.
We are to be concerned with their health by striving ourselves toward “A step ahead for healthcare”.


Our Mission
We are dedicated to providing the public with the best preventive and the most precise disease diagnosis through the diligence of our devoted experts, the guarantee of our high-quality laboratory services, and the continuation of our innovative biotechnological advancement.
Proven Technology
Molecular diagnostics technology moves fast. Newer tests tend to be quicker, more revealing and more expensive. Older tests often cost less but may not be as informative. There is a valuable role for both. Our goal is to give medical professionals and patients the widest possible choice. Our technology includes cutting edge next-generation sequencing (NGS) and other frequently used methods such as RT-PCR and Sanger sequencing.

A Step Ahead With Technology
Personalized treatments are increasingly preferred when facing many diseases and conditions. Such treatments depend on finding and interpreting individual genetic information. However, this information is often complex and can overwhelm conventional data systems.
Pangenia’s advanced test platforms can rapidly process huge amounts of genetic information within a shorter time. Our analytical instruments utilize next-generation sequencing (NGS) technology which can meticulously screen sections of an individual’s genome to identify mutations. This can be hugely effective in designing personalized treatments. We also provide real-time PCR and Sanger sequencing test services to meet patients’ differing needs.
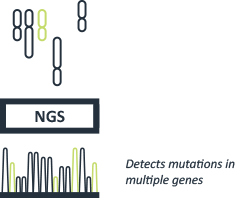
Next-Generation Sequencing (NGS)
NGS technology targets genes and mutations relevant to particular cancers. With significantly higher read length and read depth than traditional techniques, NGS allows us to sequence large genomic regions, providing comprehensive genetic profiles in a single efficient and cost-effective report. This form of testing helps identify rare somatic mutations which may have been misinterpreted as negative.
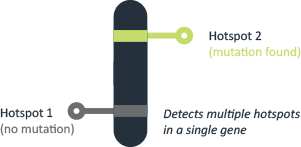
Real-time PCR
Real-time PCR testing allows us to analyze multiple targets simultaneously within a single reaction. This has a number of advantages, and can provide internal controls, lower reagent costs and preserve precious samples. Real-time PCR testing can provide reports in three to five days.
Our Team
Certifications and Accreditations
HOKLAS ISO 15189 Accreditation; Hong Kong Top Service Brand
- Pangenia Lifesciences Limited meets the requirements of ISO 15189:2022 and it has been accredited for performing specific examinations as listed in the scope of accreditation HOKLAS 862S within the test category of MEDICAL TESTING
- Awarded Hong Kong Top Service Brand 2019

HOKLAS ISO 15189 Accreditation

Hong Kong Top Service Brand 2019
Partner lab –
Knight Diagnostic Laboratories
To complement our in-house expertise, we work closely with KDL to bring oncologists a wide selection of tests. US-based KDL is a global leader in developing genetic tests for solid tumors and hematopoietic malignancies.

College of American Pathologists (CAP) accreditation – Pathology & MP















